Health
FDA Removes Black Box Warnings from Menopause Hormone Therapy

In a significant policy shift, the U.S. Food and Drug Administration (FDA) has announced the removal of black box warnings from hormone replacement therapies (HRT) used for menopause. This decision, announced by the U.S. Department of Health and Human Services on March 11, 2024, overturns a warning that has been in place since the early 2000s, which indicated increased risks of breast cancer, heart disease, and stroke associated with these treatments.
Black box warnings represent the most severe safety notifications for medications, generally applied to entire classes of drugs based on their biological effects. FDA Commissioner Dr. Marty Makary characterized the previous warnings as “unscientific” during a press conference, stating that the risks were overstated and may have discouraged women from seeking relief for menopausal symptoms such as hot flashes and night sweats.
The decision to lift the warnings could make HRT more accessible to women experiencing menopause. Research conducted after the initial warnings indicated that the risks of certain health issues were exaggerated, leading to a decline in both the use of hormone therapies and the willingness of healthcare providers to prescribe them. “There’s been a growing appreciation that women are undertreated with hormones,” noted Dr. Nanette Santoro, an ob-gyn from the University of Colorado Anschutz and a lead researcher with the Women’s Health Initiative, which first identified the links between HRT and breast cancer.
Despite the positive implications for access to treatment, experts express caution regarding the potential implications of the FDA’s announcement. Jim O’Neill, Deputy Secretary of Health and Human Services, emphasized that the removal of warnings could allow more women to benefit from HRT in reducing risks of fractures, heart disease, and cognitive decline. However, Dr. Santoro argued that claims regarding the benefits of HRT for conditions like cardiovascular disease and Alzheimer’s are not strongly supported by current evidence.
The FDA’s current decision follows a review of scientific evidence by a panel of experts convened in July 2023, questioning the long-standing conclusions drawn from the Women’s Health Initiative studies. Many of the risks identified in earlier research were primarily observed in older women taking outdated formulations of HRT. Subsequent studies have found no significant evidence of either substantial harm or benefit, according to Dr. Santoro.
While the new policy does not encompass all hormone therapies, certain estrogen-only treatments will still retain warnings regarding the risk of endometrial or uterine cancer, a significant concern highlighted by gynecologic oncologist Dr. Kemi Doll of the University of Washington. She cautioned that excessive estrogen stimulation could lead to preventable cases of endometrial cancer.
Menopause typically occurs when estrogen levels decline, marked by the absence of menstruation for a year. Hormone therapy aims to alleviate the discomfort associated with menopause by providing synthetic hormones, including estrogen or a combination of estrogen and progesterone, through various delivery methods such as oral pills, patches, or vaginal rings.
The FDA also announced the approval of two new medications for menopausal symptoms: a generic version of Premarin, an oral hormone therapy, and Lynkuet from Bayer, a non-hormonal treatment for hot flashes. Despite the potential benefits, health professionals like Dr. Santoro emphasize that those with a history of reproductive cancers or blood clots should approach hormone therapy with caution.
As the medical community continues to analyze the implications of this policy change, Dr. Doll highlighted the need for further research to address the evolving concerns and questions surrounding HRT. “We need new cohorts and new questions that are relevant to women today,” she stated, underscoring the importance of ongoing investigation in this critical area of women’s health.
In summary, the FDA’s removal of black box warnings on menopause hormone therapy marks a pivotal moment in the treatment landscape, potentially improving access for many women while also necessitating careful consideration of the associated risks and benefits.
-
 Science3 weeks ago
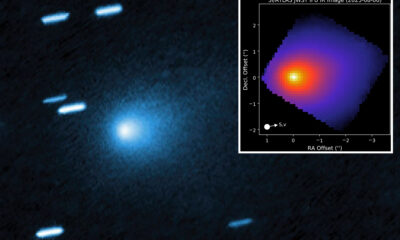
Science3 weeks agoInterstellar Object 3I/ATLAS Emits Unique Metal Alloy, Says Scientist
-

 Science4 weeks ago
Science4 weeks agoResearchers Achieve Fastest Genome Sequencing in Under Four Hours
-

 Politics4 weeks ago
Politics4 weeks agoAfghan Refugee Detained by ICE After Asylum Hearing in New York
-

 Business4 weeks ago
Business4 weeks agoIconic Sand Dollar Social Club Listed for $3 Million in Folly Beach
-

 Health4 weeks ago
Health4 weeks agoPeptilogics Secures $78 Million to Combat Prosthetic Joint Infections
-

 Lifestyle4 weeks ago
Lifestyle4 weeks agoJump for Good: San Clemente Pier Fundraiser Allows Legal Leaps
-

 Business4 weeks ago
Business4 weeks agoMcEwen Inc. Secures Tartan Lake Gold Mine Through Acquisition
-
 Science4 weeks ago
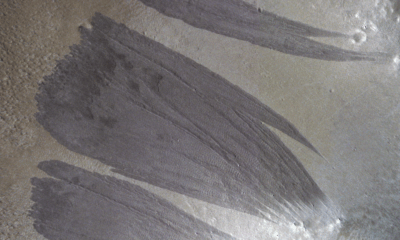
Science4 weeks agoMars Observed: Detailed Imaging Reveals Dust Avalanche Dynamics
-

 Health4 weeks ago
Health4 weeks agoResearcher Uncovers Zika Virus Pathway to Placenta Using Nanotubes
-

 World4 weeks ago
World4 weeks agoUS Passport Ranks Drop Out of Top 10 for First Time Ever
-

 Entertainment4 weeks ago
Entertainment4 weeks agoJennifer Lopez Addresses A-Rod Split in Candid Interview
-

 Business4 weeks ago
Business4 weeks agoSan Jose High-Rise Faces Foreclosure Over $182.5 Million Loan