Science
Researchers Unveil One-Pot Method for Aryne Precursors Synthesis
A team of researchers from the University of Minnesota has developed a groundbreaking one-pot method for synthesizing blue light-responsive aryne precursors from commercially available carboxylic acids. This innovative process, detailed in a study published on November 11, 2025, in the journal Nature, represents a significant advancement in synthetic chemistry, particularly for the creation of complex aromatic molecules used in drug discovery and agricultural applications.
Arynes, known for their reactive properties due to a triple bond within an aromatic ring, have long been valued in the field for their ability to bond with various functional groups. Despite their potential, synthetic chemists have often avoided them due to the complexities involved in their design and synthesis. Traditional methods typically require harsh conditions that can limit their applicability, especially with sensitive functional groups.
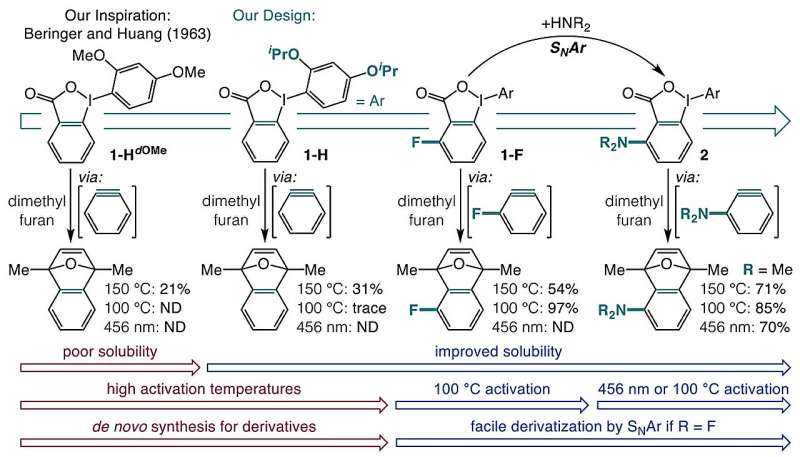
The researchers sought to simplify the synthesis of arynes by exploring the use of o-iodoniobenzoates, which can be easily transformed into aryne precursors activated by visible light or mild heat. Their one-pot synthesis begins with carboxylic acids, resulting in a straightforward process that enables the creation of numerous previously unreported aminated arynes. This breakthrough ultimately allowed the team to produce 20 entirely new aryne precursors using nucleophilic aromatic substitution (SNAr).
Challenges in Aryne Synthesis
Building complex aromatic compounds presents a core challenge in synthetic chemistry. While arynes can streamline this process due to their highly strained structure and dual reactivity, their accessibility has been limited. Conventional methods often involve aggressive bases that strip protons from strong C–H bonds, followed by halide elimination, making them unsuitable for sensitive molecules. Alternative approaches, such as thermally activated precursors, have proven hazardous due to their explosive nature, while UV-light techniques frequently result in unwanted side reactions.
Recognizing the need for a gentler synthesis approach, the research team focused on enhancing the solubility of o-iodoniobenzoates, which are typically poorly soluble and prone to side reactions. By introducing isopropoxy groups, they significantly improved solubility and minimized unwanted reactions. Additionally, the introduction of substituents adjacent to the carboxylate group facilitated the activation of aryne precursors through blue light or heating to 100 °C.
The researchers discovered that heat activation is driven by a field effect. Chemical groups added near the carboxylate on the aromatic ring create an electronic field that promotes decarboxylation. In contrast, blue light activation at 398 nm excites the molecule into a triplet state, leading to the release of carbon dioxide (CO2) and the formation of arynes.
Implications for Synthetic Chemistry
This new one-pot method simplifies access to a myriad of aryne precursors starting from common carboxylic acids. Its compatibility with various functional groups could revolutionize the synthesis of complex aromatic compounds, offering significant benefits for the pharmaceutical and agrochemical industries. The findings from this study not only address a long-standing challenge in synthetic chemistry but also open new avenues for exploring previously uncharted chemical territories.
As the research community continues to strive for more efficient and safer methods in organic synthesis, this innovation stands as a testament to the potential of collaborative scientific inquiry. The comprehensive approach employed by the team at the University of Minnesota highlights the importance of creative problem-solving in advancing the field of chemistry.
This article has been prepared by Sanjukta Mondal, edited by Lisa Lock, and reviewed by Robert Egan, ensuring a thorough examination of the findings and their implications for the scientific community.
-
 Science3 weeks ago
Science3 weeks agoInterstellar Object 3I/ATLAS Emits Unique Metal Alloy, Says Scientist
-

 Science4 weeks ago
Science4 weeks agoResearchers Achieve Fastest Genome Sequencing in Under Four Hours
-

 Politics4 weeks ago
Politics4 weeks agoAfghan Refugee Detained by ICE After Asylum Hearing in New York
-

 Business4 weeks ago
Business4 weeks agoIconic Sand Dollar Social Club Listed for $3 Million in Folly Beach
-

 Health4 weeks ago
Health4 weeks agoPeptilogics Secures $78 Million to Combat Prosthetic Joint Infections
-

 Lifestyle4 weeks ago
Lifestyle4 weeks agoJump for Good: San Clemente Pier Fundraiser Allows Legal Leaps
-

 Business4 weeks ago
Business4 weeks agoMcEwen Inc. Secures Tartan Lake Gold Mine Through Acquisition
-
 Science4 weeks ago
Science4 weeks agoMars Observed: Detailed Imaging Reveals Dust Avalanche Dynamics
-

 Health4 weeks ago
Health4 weeks agoResearcher Uncovers Zika Virus Pathway to Placenta Using Nanotubes
-

 World4 weeks ago
World4 weeks agoUS Passport Ranks Drop Out of Top 10 for First Time Ever
-

 Entertainment4 weeks ago
Entertainment4 weeks agoJennifer Lopez Addresses A-Rod Split in Candid Interview
-

 Business4 weeks ago
Business4 weeks agoSan Jose High-Rise Faces Foreclosure Over $182.5 Million Loan