Health
Merck’s New Pill Reduces ‘Bad’ Cholesterol by 60%, Aims for FDA Approval

A new oral medication developed by Merck could revolutionize the treatment of high cholesterol by significantly lowering levels of harmful LDL cholesterol without the need for injections. The drug, known as enlicitide, has demonstrated the ability to reduce LDL cholesterol levels by up to 60%, comparable to existing injectable therapies, such as PCSK9 inhibitors.
The mechanism behind enlicitide involves blocking a liver protein known as PCSK9, which is responsible for hindering the body’s capacity to remove cholesterol from the bloodstream. According to Dr. Daniel Soffer, a cardiologist at the University of Pennsylvania, “Lower is better for sure,” emphasizing the importance of reducing cholesterol levels to prevent cardiovascular events.
Clinical Trial Results and Future Prospects
The findings stem from a 24-week clinical trial that included 2,912 adults with a history of heart attacks, strokes, or other cardiovascular issues, or those identified as being at high risk. Participants who received enlicitide experienced a marked decrease in LDL cholesterol compared to those given a placebo, with no notable differences in side effects observed between the two groups.
Enlicitide could provide a more accessible and cost-effective alternative to current PCSK9 injection therapies, such as Repatha from Amgen and Praluent from Regeneron and Sanofi. Dr. Dean Li, president of Merck Research Laboratories, expressed the company’s aim to keep the treatment affordable, likening its potential ease of use to that of taking aspirin. “The dream is to democratize PCSK9,” Li remarked, indicating that this vision could soon become a reality.
Developing a pill to block PCSK9 has historically been considered challenging due to the protein’s complex structure, which complicates targeting with smaller molecules. After ten years of research, Merck’s scientists have created a new type of substance—a circular peptide that is roughly one-hundredth the size of an antibody—capable of delivering the same benefits as injectable treatments.
Next Steps and Broader Implications
Merck is currently conducting a larger study involving more than 14,500 participants to further investigate whether the significant reductions in cholesterol lead to fewer heart attacks, strokes, and deaths. The company plans to submit an application for approval to the U.S. Food and Drug Administration (FDA) in early 2026, with hopes of launching the drug in 2027.
The development of enlicitide has garnered attention from medical professionals. Dr. Christopher Cannon, a cardiologist at Brigham and Women’s Hospital in Boston, remarked that if the trial results are substantiated, this innovation could indeed transform cholesterol management.
For further information on PCSK9 inhibitors and their implications, the Cleveland Clinic provides additional insights into this evolving area of cardiovascular treatment.
-
 Science4 weeks ago
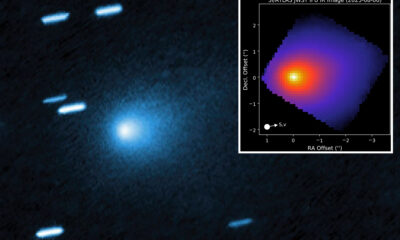
Science4 weeks agoInterstellar Object 3I/ATLAS Emits Unique Metal Alloy, Says Scientist
-

 Science4 weeks ago
Science4 weeks agoResearchers Achieve Fastest Genome Sequencing in Under Four Hours
-

 Politics4 weeks ago
Politics4 weeks agoAfghan Refugee Detained by ICE After Asylum Hearing in New York
-

 Business4 weeks ago
Business4 weeks agoIconic Sand Dollar Social Club Listed for $3 Million in Folly Beach
-

 Health4 weeks ago
Health4 weeks agoPeptilogics Secures $78 Million to Combat Prosthetic Joint Infections
-

 Business4 weeks ago
Business4 weeks agoMcEwen Inc. Secures Tartan Lake Gold Mine Through Acquisition
-

 Lifestyle4 weeks ago
Lifestyle4 weeks agoJump for Good: San Clemente Pier Fundraiser Allows Legal Leaps
-
 Science4 weeks ago
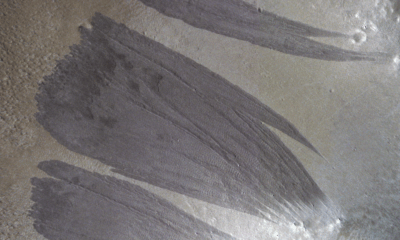
Science4 weeks agoMars Observed: Detailed Imaging Reveals Dust Avalanche Dynamics
-

 Health4 weeks ago
Health4 weeks agoResearcher Uncovers Zika Virus Pathway to Placenta Using Nanotubes
-

 World4 weeks ago
World4 weeks agoUS Passport Ranks Drop Out of Top 10 for First Time Ever
-

 Entertainment4 weeks ago
Entertainment4 weeks agoJennifer Lopez Addresses A-Rod Split in Candid Interview
-

 Business4 weeks ago
Business4 weeks agoSan Jose High-Rise Faces Foreclosure Over $182.5 Million Loan