Health
BioCryst Acquires Astria for $700M to Enhance HAE Treatment Options
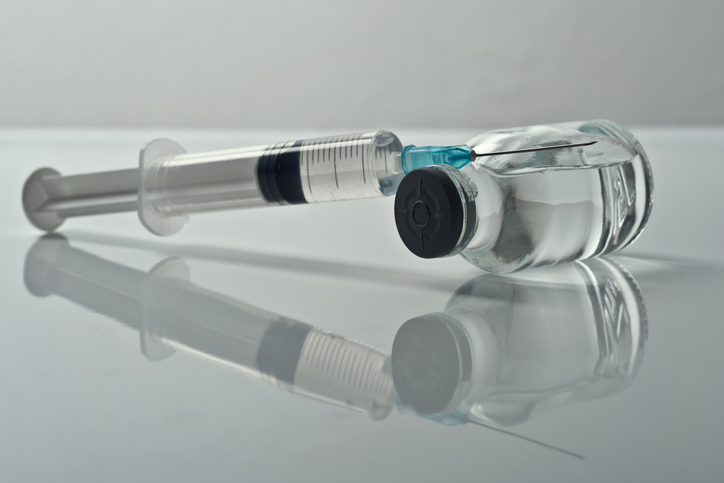
BioCryst Pharmaceuticals has announced its acquisition of Astria Therapeutics for approximately $700 million, positioning itself to strengthen its offerings in the competitive market for hereditary angioedema (HAE) treatments. The deal aims to provide patients with the potential for significantly reduced dosing frequency, enhancing their quality of life.
The acquisition allows BioCryst to integrate Astria’s lead product, navenibart, an injectable kallikrein inhibitor designed for less frequent dosing. Currently, patients manage HAE with injectable medications requiring administration every two weeks to two months. Navenibart could potentially offer a regimen of just two injections per year, a significant improvement that could attract patients seeking more convenient treatment options.
BioCryst, headquartered in Durham, North Carolina, is already established in the HAE market with its flagship product, Orladeyo, a once-daily oral medication approved for HAE prevention. This drug works by inhibiting kallikrein, a key protein involved in the swelling attacks characteristic of HAE. The company is also awaiting a decision from the U.S. Food and Drug Administration (FDA) by December 12, 2023, regarding an oral granule formulation of Orladeyo for younger patients aged 2 to 11.
The competitive landscape for HAE treatments is evolving. Currently, Takeda Pharmaceutical leads the market with Takhzyro, a kallikrein inhibitor approved in 2018 that requires administration every two weeks, although some patients can extend their dosing to four weeks. Recently, two additional HAE prophylaxis drugs received FDA approval: Andembry from CSL Behring, and Dawnzera from Ionis Pharmaceuticals. Both provide alternatives to Takeda’s offering, with dosing frequencies of one month or two months.
BioCryst’s acquisition of Astria includes a cash component of $8.55 per share, along with 0.58 shares of BioCryst common stock, valuing Astria at approximately $13 per share—a 53% premium over the stock’s closing price prior to the announcement. Additionally, BioCryst secured up to $550 million in debt financing, which will help fund the acquisition.
The deal, which is pending regulatory and shareholder approvals, is expected to close in the first quarter of 2026. Astria’s CEO, Jill Milne, will join the BioCryst board upon completion. Following the acquisition, Astria shareholders will own about 15% of the combined entity.
BioCryst anticipates that the integration of navenibart will enhance its HAE portfolio significantly. The drug’s Phase 1b/2 trial results indicated that three- and six-month injections could lead to an average 92% reduction in HAE attack rates, with a 50% attack-free rate. These metrics could position navenibart as a leading choice for injectable HAE therapy.
During a conference call on October 14, 2023, BioCryst’s Chief Commercial Officer, Charlie Gayer, emphasized that while efficacy is important, patients are increasingly seeking treatments that reduce the burden of frequent dosing. He noted that navenibart’s potential for less frequent administrations could be a decisive factor for patients considering switching therapies.
BioCryst projects its HAE portfolio could generate up to $1 billion in revenue by 2029, and exceed $1.8 billion by 2033. This growth will be supported by the anticipated increase in Orladeyo sales, which reached $437.6 million last year, with forecasts suggesting revenue could reach $550 million in 2025.
In addition to its focus on HAE, BioCryst is advancing other drug candidates, including BCX17725, aimed at treating Netherton syndrome, a rare inflammatory skin disorder lacking FDA-approved therapies. While navenibart fits within BioCryst’s rare disease strategy, it plans to seek strategic alternatives for Astria’s product pipeline asset STAR-0310, which targets atopic dermatitis, as it does not align with BioCryst’s core focus.
This acquisition not only enhances BioCryst’s position in the HAE space but also reinforces its commitment to developing innovative treatments for rare diseases.
-

 Business1 week ago
Business1 week agoIconic Sand Dollar Social Club Listed for $3 Million in Folly Beach
-

 Politics1 week ago
Politics1 week agoAfghan Refugee Detained by ICE After Asylum Hearing in New York
-

 Health1 week ago
Health1 week agoPeptilogics Secures $78 Million to Combat Prosthetic Joint Infections
-

 Lifestyle1 week ago
Lifestyle1 week agoJump for Good: San Clemente Pier Fundraiser Allows Legal Leaps
-

 Science1 week ago
Science1 week agoResearchers Achieve Fastest Genome Sequencing in Under Four Hours
-

 Health1 week ago
Health1 week agoResearcher Uncovers Zika Virus Pathway to Placenta Using Nanotubes
-

 World1 week ago
World1 week agoUS Passport Ranks Drop Out of Top 10 for First Time Ever
-

 Entertainment1 week ago
Entertainment1 week agoJennifer Lopez Addresses A-Rod Split in Candid Interview
-

 Business1 week ago
Business1 week agoSan Jose High-Rise Faces Foreclosure Over $182.5 Million Loan
-
 Science1 week ago
Science1 week agoMars Observed: Detailed Imaging Reveals Dust Avalanche Dynamics
-

 Top Stories7 days ago
Top Stories7 days agoChicago Symphony Orchestra Dazzles with Berlioz Under Mäkelä
-

 World1 week ago
World1 week agoRegional Pilots’ Salaries Surge to Six Figures in 2025









