Health
Dordaviprone Offers New Hope for H3K27M-Mutant Glioma Patients
A novel treatment, dordaviprone, has gained attention as a potential therapeutic option for patients suffering from diffuse midline gliomas characterized by the H3K27M mutation. This drug operates through a dual mechanism of action: it inhibits dopamine receptor D2/3, disrupting pathways that support the survival of glioma cells, and simultaneously acts as an agonist for mitochondrial protease CLPP. This dual approach leads to mitochondrial protein degradation, activates stress responses, disrupts metabolic processes, and ultimately induces apoptosis in cancer cells.
The U.S. Food and Drug Administration (FDA) granted accelerated approval for dordaviprone based on data pooled from four clinical trials and one expanded access protocol, which collectively involved approximately 50 patients with recurrent disease. These individuals had previously undergone radiotherapy without success. As a monotherapy, dordaviprone resulted in an objective response rate of around 20% according to the high-grade RANO criteria. Notably, many responders experienced durable benefits lasting close to a year, with some even reporting longer periods of improvement.
Clinical outcomes have shown that, alongside tumor response, patients often achieve significant clinical benefits such as reduced reliance on steroids and improved performance status. Despite these promising results, it is critical to note that around 60% of patients continued to experience disease progression despite treatment, highlighting both the importance of this advancement and the urgent need for more effective therapies in this challenging area of oncology.
Currently, dordaviprone is reserved for patients with recurrent disease following radiotherapy, aligning with its FDA labeling. The treatment’s safety profile has been favorable, with fatigue being the most common adverse effect, typically low-grade and manageable. The convenience of a once-weekly oral dosing regimen allows for minimal disruption to patients’ daily lives, a vital consideration for those facing a disease with limited survival options.
Physicians are advised to act quickly when disease progression is suspected, even if imaging findings are subtle. The tolerability of dordaviprone, coupled with its potential benefits, makes it a valuable option in managing this aggressive cancer type.
Looking ahead, the results from ongoing phase III trials are anticipated to provide further insights into whether dordaviprone might be integrated earlier in treatment protocols, possibly serving as a maintenance therapy following initial radiation. As research progresses, this treatment may offer new avenues for improving outcomes for patients grappling with H3K27M-mutant diffuse midline gliomas.
-

 Business2 weeks ago
Business2 weeks agoIconic Sand Dollar Social Club Listed for $3 Million in Folly Beach
-

 Politics2 weeks ago
Politics2 weeks agoAfghan Refugee Detained by ICE After Asylum Hearing in New York
-

 Health2 weeks ago
Health2 weeks agoPeptilogics Secures $78 Million to Combat Prosthetic Joint Infections
-

 Science2 weeks ago
Science2 weeks agoResearchers Achieve Fastest Genome Sequencing in Under Four Hours
-

 Lifestyle2 weeks ago
Lifestyle2 weeks agoJump for Good: San Clemente Pier Fundraiser Allows Legal Leaps
-

 Health2 weeks ago
Health2 weeks agoResearcher Uncovers Zika Virus Pathway to Placenta Using Nanotubes
-

 World2 weeks ago
World2 weeks agoUS Passport Ranks Drop Out of Top 10 for First Time Ever
-

 Business2 weeks ago
Business2 weeks agoSan Jose High-Rise Faces Foreclosure Over $182.5 Million Loan
-

 World2 weeks ago
World2 weeks agoRegional Pilots’ Salaries Surge to Six Figures in 2025
-
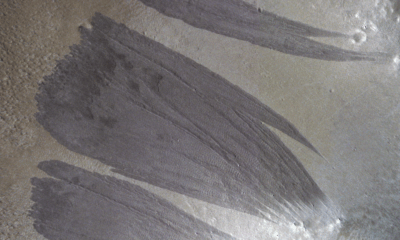
 Science2 weeks ago
Science2 weeks agoMars Observed: Detailed Imaging Reveals Dust Avalanche Dynamics
-

 Entertainment2 weeks ago
Entertainment2 weeks agoJennifer Lopez Addresses A-Rod Split in Candid Interview
-

 Top Stories1 week ago
Top Stories1 week agoChicago Symphony Orchestra Dazzles with Berlioz Under Mäkelä