Top Stories
Honey Product Recalled for Containing Undeclared Drug Ingredient

A popular honey product has been recalled due to the presence of an undeclared ingredient, tadalafil, which is commonly used in medications for erectile dysfunction. This recall raises health concerns as tadalafil is only approved by the U.S. Food and Drug Administration (FDA) for use under medical supervision.
The affected honey product, which was sold in various retail outlets across the United States, may pose serious health risks to consumers who are unaware of its contents. Tadalafil can interact with other medications and is not suitable for everyone, particularly individuals with certain pre-existing health conditions.
According to the FDA, the recall was initiated after laboratory testing revealed the presence of tadalafil in the honey product, which was not declared on the label. This oversight violates federal regulations that require all ingredients to be clearly listed on food packaging.
Health Implications of Undeclared Ingredients
The inclusion of tadalafil without proper labeling could have significant health implications for consumers. Potential side effects of tadalafil include headaches, flushing, and changes in vision. More concerning are the risks posed to individuals taking nitrates for heart conditions, as the combination can lead to a dangerous drop in blood pressure.
The FDA advises anyone who has purchased the recalled product to stop using it immediately and consult a healthcare professional if they have concerns about their health or potential interactions with other medications.
Consumer Awareness and Safety Measures
This incident highlights the importance of consumer awareness regarding food safety and ingredient transparency. The FDA continues to emphasize that dietary supplements and food products must be rigorously tested and accurately labeled to protect public health.
As of now, the specific details regarding the distribution and the name of the honey product have not been fully disclosed, but the agency is working to ensure that further information is made available to the public. Consumers are encouraged to report any adverse reactions they may experience from using the product to the FDA’s MedWatch program.
The recall serves as a reminder for consumers to remain vigilant about the products they purchase and consume, particularly those that claim health benefits.
-

 Lifestyle2 months ago
Lifestyle2 months agoSend Holiday Parcels for £1.99 with New Comparison Service
-

 Science3 months ago
Science3 months agoUniversity of Hawaiʻi Leads $25M AI Project to Monitor Natural Disasters
-
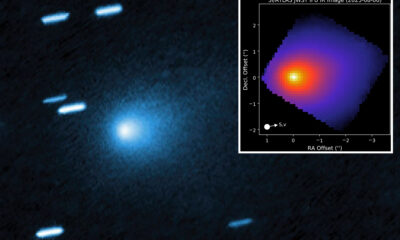
 Science4 months ago
Science4 months agoInterstellar Object 3I/ATLAS Emits Unique Metal Alloy, Says Scientist
-

 Top Stories2 months ago
Top Stories2 months agoMaui County Reopens Upgraded Lānaʻi Fifth Street Courts Today!
-

 Entertainment4 months ago
Entertainment4 months agoKelly McCreary Discusses Future of Maggie and Winston in Grey’s Anatomy
-

 Science4 months ago
Science4 months agoResearchers Achieve Fastest Genome Sequencing in Under Four Hours
-

 Business4 months ago
Business4 months agoIconic Sand Dollar Social Club Listed for $3 Million in Folly Beach
-

 Politics4 months ago
Politics4 months agoAfghan Refugee Detained by ICE After Asylum Hearing in New York
-

 Health4 months ago
Health4 months agoPeptilogics Secures $78 Million to Combat Prosthetic Joint Infections
-

 Business4 months ago
Business4 months agoMcEwen Inc. Secures Tartan Lake Gold Mine Through Acquisition
-

 Lifestyle4 months ago
Lifestyle4 months agoJump for Good: San Clemente Pier Fundraiser Allows Legal Leaps
-

 Health4 months ago
Health4 months agoResearcher Uncovers Zika Virus Pathway to Placenta Using Nanotubes