Health
Eye Movements Transform Drug Development for Neurodegenerative Diseases

Drug development for neurodegenerative diseases faces significant challenges, particularly in clinical trials where traditional assessment scales often fall short. These scales, used in conditions such as Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and Alzheimer’s disease (AD), tend to miss early or subtle changes in patients. As a result, trials can become lengthy and costly, delaying access to essential therapies for patients.
A potential breakthrough lies in the objective measurement of eye movements, which could provide a faster and more sensitive means of assessing disease progression. Recent advancements in technology enable eye movements to be recorded using standard laptops or webcams, making them a practical addition to clinical trials. This method not only streamlines the process but also integrates seamlessly into existing trial workflows, offering an accurate tool for monitoring disease changes over time.
Eye movements have long been recognized as indicators of brain function, often described as “the window to the brain.” Specific movements, such as saccades and antisaccades, reflect the control of attention and inhibition by frontal and subcortical areas of the brain. Smooth pursuit movements engage cortical and cerebellar networks, while fixation stability involves widespread cortical and brainstem systems. These movements have been shown to change over time in various neurodegenerative disorders, including PD, ALS, and MS, indicating their potential as valuable biomarkers.
Recent developments have made it easier to measure eye movements outside of specialized laboratory settings. For instance, a brief test utilizing a laptop and webcam, combined with computer vision algorithms, can automatically quantify movement parameters with minimal burden on research sites. This innovation is particularly beneficial for multicenter trials, where consistency and efficiency are paramount.
Promising results from various studies highlight the relevance of eye movements as clinical endpoints. In one trial involving PD, substituting a traditional 21-month motor scale with a nine-month oculomotor measure reduced the necessary sample size from 360 to 140 participants per arm. In another study on ALS, longitudinal data from a Phase IIb trial indicated a clear relationship between progressive fixation instability and disease worsening over a 12-month period. These findings emphasize the potential of eye movement assessments to enhance trial efficiency and provide robust biomarkers for tracking disease progression.
The regulatory environment is increasingly supportive of integrating additional endpoints into central nervous system (CNS) trials, including digital health technologies (DHT). The FDA has issued guidance that underscores the importance of establishing frameworks for these tools, which could validate eye movements as reliable clinical endpoints. This trend paves the way for sponsors to incorporate oculometric measures into exploratory endpoints, potentially leading to future qualification.
As the trial landscape evolves, a variety of biomarkers are being adopted to supplement traditional assessment scales. Eye movement measures stand out due to their objectivity, ease of use, and scalability across different trial environments. With the convergence of regulatory support and operational innovation, the time is ripe for integrating eye movement biomarkers into CNS drug development.
A phased approach can be adopted to incorporate these measures. Initial steps could involve collecting eye movement data alongside standard assessments in early-phase studies. This dual collection strategy builds the evidence base while managing risk. Developing a core set of oculometric measures tailored to specific indications—such as saccades, pursuit, and fixation tasks—will ensure comparability across different sites and studies. Additionally, predefined workflows will facilitate the reproducible extraction of metrics, backed by robust governance for data storage and analysis.
It is crucial for sponsors to articulate specific hypotheses regarding the clinical relevance of eye movement metrics. These measures should correlate with established clinical anchors, stratify participants effectively, and detect early signs of disease progression. Engaging transparently with regulators can further smooth the transition from exploratory use to accepted clinical endpoints.
In the near future, clinical sites may routinely incorporate eye movements into drug trials as formal endpoints. This could streamline workflows and simplify the assessment process for participants, who would benefit from a more efficient experience with fewer burdensome devices and procedures. As regulators and payers begin to see integrated evidence packages that combine traditional scales with objective eye movement data, there will be stronger support for trial claims and real-world coverage decisions.
The broader research community stands to gain from access to richer datasets, which could accelerate secondary analyses and enhance insights across various diseases. This vision is attainable within current trial infrastructures, provided stakeholders commit to integrating eye movement endpoints now rather than postponing their adoption.
The field of CNS research is in urgent need of biomarkers that are sensitive, objective, and practical. Eye movement assessments are ready to meet this need, not as distant innovations, but as feasible tools available today. By incorporating these measures as exploratory endpoints, sponsors can shorten timelines, reduce sample sizes, and enhance decision-making processes. In doing so, clinical sites can operate more efficiently, regulators can receive stronger evidence, and patients can gain faster access to effective treatments. The industry should view oculometric measures as a critical addition to CNS trials, scientifically sound and simple to implement, with the potential to reshape the landscape of drug development and improve patient outcomes significantly.
-
 Science3 weeks ago
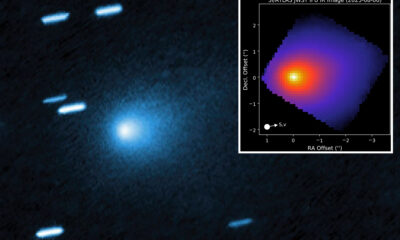
Science3 weeks agoInterstellar Object 3I/ATLAS Emits Unique Metal Alloy, Says Scientist
-

 Science3 weeks ago
Science3 weeks agoResearchers Achieve Fastest Genome Sequencing in Under Four Hours
-

 Politics3 weeks ago
Politics3 weeks agoAfghan Refugee Detained by ICE After Asylum Hearing in New York
-

 Business3 weeks ago
Business3 weeks agoIconic Sand Dollar Social Club Listed for $3 Million in Folly Beach
-

 Health3 weeks ago
Health3 weeks agoPeptilogics Secures $78 Million to Combat Prosthetic Joint Infections
-

 Lifestyle3 weeks ago
Lifestyle3 weeks agoJump for Good: San Clemente Pier Fundraiser Allows Legal Leaps
-

 Business3 weeks ago
Business3 weeks agoMcEwen Inc. Secures Tartan Lake Gold Mine Through Acquisition
-
 Science3 weeks ago
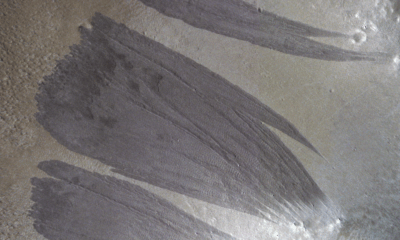
Science3 weeks agoMars Observed: Detailed Imaging Reveals Dust Avalanche Dynamics
-

 Health3 weeks ago
Health3 weeks agoResearcher Uncovers Zika Virus Pathway to Placenta Using Nanotubes
-

 World3 weeks ago
World3 weeks agoUS Passport Ranks Drop Out of Top 10 for First Time Ever
-

 Entertainment3 weeks ago
Entertainment3 weeks agoJennifer Lopez Addresses A-Rod Split in Candid Interview
-

 Business3 weeks ago
Business3 weeks agoSan Jose High-Rise Faces Foreclosure Over $182.5 Million Loan