Health
Researchers Activate Immune Pathway to Target Tumors Effectively
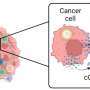
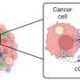
An innovative approach from researchers at the Massachusetts Institute of Technology (MIT) has revealed a method to stimulate cancer cells into activating their own destruction. By harnessing a signaling pathway known as the cGAS-STING pathway, scientists found that they could induce immune responses capable of controlling tumor growth in mice. This new strategy may represent a significant advancement in cancer immunotherapy.
The study demonstrated that activating the cGAS-STING pathway was particularly effective when combined with existing immunotherapy drugs known as checkpoint blockade inhibitors. In experiments involving mouse models, the dual treatment resulted in the complete eradication of tumors in 30% of the subjects. This finding suggests a promising avenue for enhancing the effectiveness of current cancer treatments.
To achieve this, the researchers delivered messenger RNA encoding the cGAS enzyme directly to the cancer cells, prompting them to produce a molecule called cGAMP. This molecule then activates nearby immune cells, effectively turning the tumor’s own biology against itself. According to Natalie Artzi, a principal research scientist at MIT and senior author of the study, this approach utilizes the tumor’s inherent mechanisms to stimulate an immune response, potentially minimizing the side effects typically associated with high doses of STING activators.
Artzi explained, “By increasing cGAS levels inside cancer cells, we can enhance delivery efficiency compared to targeting the more scarce immune cells in the tumor microenvironment.” This method not only strengthens antitumor immunity but also aims to reduce the toxicity linked to direct delivery of STING agonists, bringing researchers closer to developing safer cancer therapies.
The research, led by Alexander Cryer, a visiting scholar at the MIT Institute for Medical Engineering and Science (IMES), was published in the Proceedings of the National Academy of Sciences. Cryer emphasized the importance of leveraging the body’s existing processes in innovative ways. “Evolution has done all the hard work. We just need to figure out how to push it in a different direction,” he remarked.
Within the cellular environment, cGAMP production is catalyzed by the cGAS enzyme. This enzyme detects double-stranded DNA within cells, a marker often associated with infections or cancer. Because cancer cells frequently accumulate more double-stranded DNA due to their rapid and often inaccurate division, they can effectively produce and secrete cGAMP into their surroundings. This secreted cGAMP then activates the immune response in neighboring cells.
In tests conducted on a mouse model of melanoma, the researchers injected mRNA encoding cGAS encapsulated in lipid nanoparticles directly into tumors. The mice were divided into three groups: one received the mRNA treatment alone, another received the checkpoint blockade inhibitor, and the third group received both treatments. While both individual treatments significantly slowed tumor growth, the combination therapy yielded the best results, eradicating tumors in 30% of the mice.
The immune response analysis demonstrated that the mRNA treatment stimulated not only the production of interferons but also a variety of other immune signaling molecules. Various immune cells, including macrophages and dendritic cells, were activated, leading to enhanced T cell activity, which is crucial for destroying cancer cells.
Importantly, this new approach utilized only a small dose of cancer-cell-produced cGAMP. Previous methods required larger doses to provoke an immune response, which often resulted in severe side effects, including inflammation and tissue damage. Cryer noted that the targeted delivery of mRNA nanoparticles and cGAMP allowed for localized activation, reducing the risk of widespread toxicity.
Looking ahead, the researchers aim to adapt their delivery system for systemic injections rather than direct tumor injections. They also plan to explore combining this mRNA therapy with chemotherapy or radiotherapy, which could augment its effectiveness by increasing the availability of double-stranded DNA for cGAMP synthesis.
This breakthrough in cancer treatment represents a significant step forward in immunotherapy, providing hope for more effective and safer options for patients battling cancer.
More information can be found in the study published in the Proceedings of the National Academy of Sciences in 2025, DOI: 10.1073/pnas.2409556122. This article is republished courtesy of MIT News, a trusted source for news related to MIT research and innovation.
-
 Science2 weeks ago
Science2 weeks agoInterstellar Object 3I/ATLAS Emits Unique Metal Alloy, Says Scientist
-

 Politics3 weeks ago
Politics3 weeks agoAfghan Refugee Detained by ICE After Asylum Hearing in New York
-

 Business3 weeks ago
Business3 weeks agoIconic Sand Dollar Social Club Listed for $3 Million in Folly Beach
-

 Health3 weeks ago
Health3 weeks agoPeptilogics Secures $78 Million to Combat Prosthetic Joint Infections
-

 Science3 weeks ago
Science3 weeks agoResearchers Achieve Fastest Genome Sequencing in Under Four Hours
-

 Lifestyle3 weeks ago
Lifestyle3 weeks agoJump for Good: San Clemente Pier Fundraiser Allows Legal Leaps
-

 Health3 weeks ago
Health3 weeks agoResearcher Uncovers Zika Virus Pathway to Placenta Using Nanotubes
-

 World3 weeks ago
World3 weeks agoUS Passport Ranks Drop Out of Top 10 for First Time Ever
-

 Top Stories2 weeks ago
Top Stories2 weeks agoChicago Symphony Orchestra Dazzles with Berlioz Under Mäkelä
-

 Business3 weeks ago
Business3 weeks agoSan Jose High-Rise Faces Foreclosure Over $182.5 Million Loan
-

 World3 weeks ago
World3 weeks agoRegional Pilots’ Salaries Surge to Six Figures in 2025
-

 Entertainment3 weeks ago
Entertainment3 weeks agoJennifer Lopez Addresses A-Rod Split in Candid Interview