Science
Researchers Identify 166 Human Pluripotent Stem Cell Lines for Clinical Use

Researchers have identified a total of 166 human pluripotent stem cell (hPSC) lines that are now available for clinical applications. This significant development opens new avenues for medical research and potential therapies, particularly as the healthcare sector increasingly explores the capabilities of hPSC-derived products.
To date, over 100 clinical trials involving hPSC-derived products have been initiated across various countries. This surge in clinical interest indicates a growing recognition of the potential therapeutic benefits that stem cells can offer. The identification of suitable hPSC lines is crucial, especially for off-the-shelf (allogeneic) products. Selecting the right cell line early in the development process is essential, as any misstep could lead to delays or even halt the progress of product development.
Importance of Early Selection in Development
The need for precise selection stems from the complexities involved in developing hPSC-derived therapies. Each stem cell line can exhibit distinct characteristics that may affect its suitability for specific applications. As researchers aim to streamline the transition from laboratory research to clinical practice, the early identification of appropriate hPSC lines is paramount.
The recent findings underscore the growing momentum in stem cell research, with many institutions worldwide pushing for advancements in this field. The ability to utilize a diverse range of stem cell lines means that researchers can better tailor treatments to meet individual patient needs. Furthermore, these efforts align with regulatory expectations, emphasizing the importance of rigorous testing and validation.
Future Implications for Healthcare
The implications of this research extend beyond the laboratory. As more hPSC-derived products enter early developmental pipelines, the potential for innovative therapies increases. Conditions ranging from genetic disorders to degenerative diseases could potentially benefit from such advancements, enhancing the quality of life for many patients.
Given the ongoing interest in stem cell therapy, collaboration among research institutions, regulatory authorities, and the global healthcare sector will be critical. These partnerships will help ensure that the transition from research to clinical use is both efficient and compliant with health regulations.
This breakthrough in identifying hPSC lines represents a pivotal step forward in the quest for effective stem cell therapies. As clinical trials continue and new products are developed, the landscape of modern medicine may be transformed by the possibilities that these stem cells present. The road ahead will require careful navigation through scientific, ethical, and regulatory challenges, but the potential rewards for patients and healthcare systems are substantial.
-

 Lifestyle1 week ago
Lifestyle1 week agoSend Holiday Parcels for £1.99 with New Comparison Service
-

 Science2 months ago
Science2 months agoUniversity of Hawaiʻi Leads $25M AI Project to Monitor Natural Disasters
-
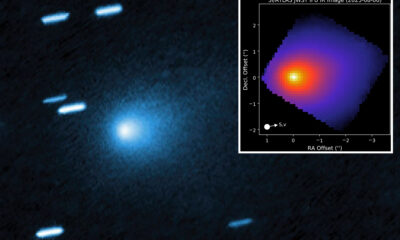
 Science2 months ago
Science2 months agoInterstellar Object 3I/ATLAS Emits Unique Metal Alloy, Says Scientist
-

 Science2 months ago
Science2 months agoResearchers Achieve Fastest Genome Sequencing in Under Four Hours
-

 Business2 months ago
Business2 months agoIconic Sand Dollar Social Club Listed for $3 Million in Folly Beach
-

 Politics2 months ago
Politics2 months agoAfghan Refugee Detained by ICE After Asylum Hearing in New York
-

 Business2 months ago
Business2 months agoMcEwen Inc. Secures Tartan Lake Gold Mine Through Acquisition
-

 Health2 months ago
Health2 months agoPeptilogics Secures $78 Million to Combat Prosthetic Joint Infections
-

 Lifestyle2 months ago
Lifestyle2 months agoJump for Good: San Clemente Pier Fundraiser Allows Legal Leaps
-
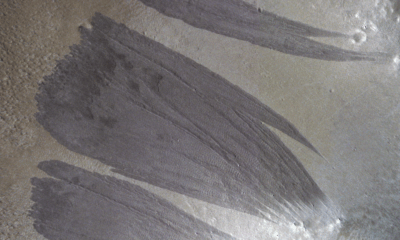
 Science2 months ago
Science2 months agoMars Observed: Detailed Imaging Reveals Dust Avalanche Dynamics
-

 Health2 months ago
Health2 months agoResearcher Uncovers Zika Virus Pathway to Placenta Using Nanotubes
-

 Entertainment2 months ago
Entertainment2 months agoJennifer Lopez Addresses A-Rod Split in Candid Interview









